A new original research article in the scientific journal Pigment Cell Melanoma Research has been published by the Membrane Traffic in Disease laborator. This study is led by researcher Duarte Barral with Michael Hall as first author, in collaboration with Abel Oliva from the Biomolecular Dignostics lab at ITQB NOVA. It explores the use of 3D models of human skin as an alternative to animal models and invasive biopsies. We asked the authors about the potential of this new methodology.
 Michael Hall, João Charneca, Matilde Neto and Duarte Barral
Michael Hall, João Charneca, Matilde Neto and Duarte Barral
What discoveries led you to the research described in your publication?
Our lab has been working with 3D skin models, which we developed in collaboration with Abel Oliva's group at ITQB-NOVA. In addition to having the same structural features of human skin, we noticed that with the addition of a very small number of melanin pigment-producing cells, i.e., melanocytes, these lab-grown ‘skins’ quickly and efficiently disperses melanin throughout the layer representing the epidermis. This is strikingly similar to what happens in our skin, and it is this process that gives rise to different skin tones between individuals and protects us from potentially harmful UV irradiation from the sun.
What were you trying to understand and what is the main discovery of this work?
We wanted to understand how our 3D skin models achieve this efficient pigment dispersion, and whether this occurs in the same way as we previously observed in human skin samples. To approach this question, we performed in-depth microscopical analyses to demonstrate that the pigment-producing cells (melanocytes) grow long protrusions (‘branches’) through the epidermis to efficiently transfer melanin to the pigment-storing cells (keratinocytes). We also showed that melanin is transferred from the melanocytes to the keratinocytes in the same way as we previously observed in human skin biopsies.
Why is this important?
This work demonstrates that our lab-grown 3D skin models are strikingly comparable to human skin, both in structure and the mechanism by which pigmentation is achieved. This will provide a valuable model for disease modelling and drug/cosmetic testing, whilst reducing the reliance on animal models and invasive skin biopsies.
Can you use an analogy to help us understand your work?
In both human skin and our lab-grown skin models, the pigment-producing melanocytes grow long ‘branches’ throughout the epidermis and secrete melanin from these branches. Then, keratinocytes engulf, ingest, and store the melanin for long periods to achieve skin pigmentation. This is akin of the process used by cells of our immune system to ingest microorganisms, but melanin is not destroyed, contrary to what generally happens with the microorganisms. This is something that we are trying to understand, as well.
What questions remain to be asked?
We do not know whether other mechanisms of pigment transfer might co-exist in stimulated, diseased, or stressed conditions, such as when our skin is exposed to the sunlight and tans. This is something that we are currently investigating.
The original article is titled "Reconstructed human pigmented skin/epidermis models achieve epidermal pigmentation through melanocore transfer" and is available online at the Pigment Cell Melanoma Research journal.
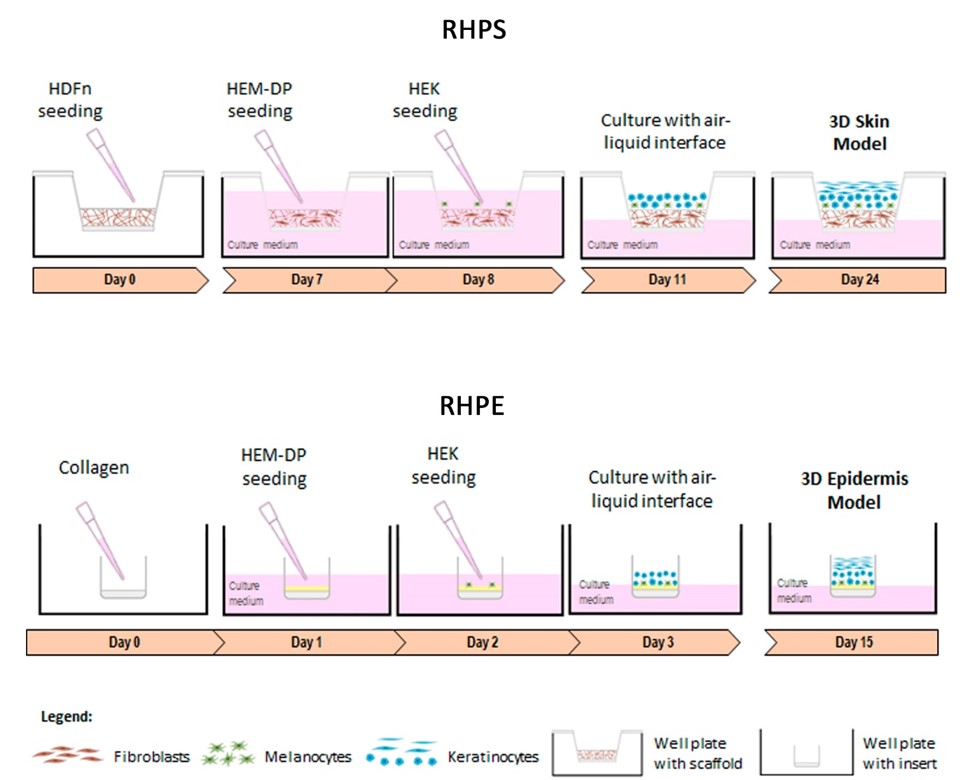
Schematic representation depicting the stages of forming reconstructed human pigmented skin and epidermis. RHPS: On day 0, fibroblasts (neonatal human dermal fibroblasts, HDFn) are seeded onto the scaffold to allow the formation of the dermis. On day 7, the formation of the epidermis begins with the addition of melanocytes (human epidermal melanocytes from darkly pigmented skin, HEM-DP) followed by addition of keratinocytes (neonatal human epidermal keratinocytes, HEKn) on the following day. Three days after, apical media is removed to achieve the air-liquid interface and on day 24, the model is fully formed. RHPE: The epidermis model is formed on a collagen-coated membrane to support melanocytes and keratinocytes. On day 1, the melanocytes are seeded, followed by keratinocytes on the following day. One day after, the air-liquid interface is established and on day 15 the epidermis is completed.