A groundbreaking study led by a team of researchers from Otília Vieira’s Lysosomes and Disease lab at NMS Research, has revealed a novel mechanism underlying the progression of atherosclerosis, a leading cause of cardiovascular diseases worldwide.

José Ramalho, Otília Vieira & André Marques
Atherosclerosis, the buildup of plaque within arterial walls, is driven by the oxidation of low-density lipoproteins (LDL) and subsequent lipid accumulation in macrophages, resulting in the formation of foam cells. The research team, building upon their prior identification of a family of oxidized lipids called cholesteryl hemiesters (ChE) in plasma and atherosclerotic lesions, set out to investigate the impact of a specific ChE called cholesteryl hemiazelate on macrophages.
The researchers discovered that exposure to cholesteryl hemiazelate led to the formation of enlarged lysosomes, cell components known as “cell recycling centers”, in macrophages, filled with undigested lipids and cholesterol esters. These dysfunctional lysosomes were found to be more prone to exocytosis, releasing their undigested contents into the extracellular environment. This process has potential negative implications for atherosclerosis progression.
Furthermore, the study identified the involvement of a family of transcription factors (MiT/TFE), proteins involved in gene regulation, in the molecular mechanism underlying this transformation. Remarkably, the presence of these factors protected macrophages from lipid-induced cytotoxicity.
"The findings from our research shed new light on the pathogenesis of atherosclerosis and offer insights into potential diagnostic and therapeutic avenues," said André R. A. Marques, one of the first authors of the study.
The research was published on Traffic journal and was selected as the cover of the July issue.
The oxidation of LDL has long been recognized as a key driver of atherosclerosis. However, the complex composition of oxidized LDL, comprising over 3000 bioactive lipids, necessitates a deeper understanding of the specific lipid species responsible for triggering pathological effects. Unraveling the role of cholesteryl hemiazelate and related oxidized lipids will aid in the development of early diagnostic tools and targeted therapies for cardiovascular diseases.
To elucidate the full scope of the impact of these oxidized lipids, further investigation is required. The researchers aim to explore whether other lipids in the ChE family exhibit similar or distinct effects on atherosclerosis. Additionally, they plan to examine the correlation between ChE levels in the blood and the likelihood of developing cardiovascular diseases, potentially paving the way for powerful pre-emptive diagnostic tools and prognostic markers.
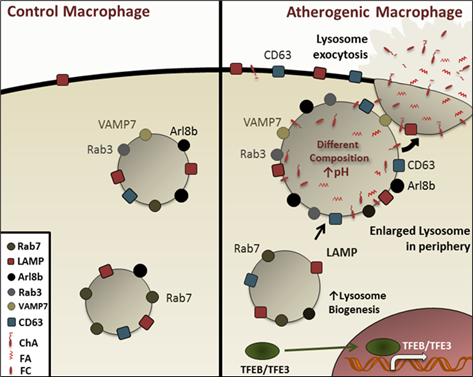
Schematic representation of the enlarged peripheral lysosomes induced by ChA in macrophages. These
lipid-loaded lysosomes present a different membrane protein composition, are dysfunctional and more peripheral when compared to lysosomes from control cells. Upon ChA treatment, there is an accumulation of undigested cargo and through the recruitment of Arl8b and Rab3a the lysosomes are translocated to the cell periphery. Here, we report that these enlarged and malfunction lysosomes present molecular machinery characteristic of secretory lysosomes, CD63 and VAMP7, becoming more prone to fuse with the plasma membrane. Consequently, the undigested and inflammatory content is released in the extracellular environment, thus contributing to the atheroma progression.
Moreover, the study opens up possibilities for therapeutic interventions by demonstrating the potential to reverse the detrimental effects of cholesteryl hemiazelate by "regenerating" dysfunctional lysosomes. Future research will delve into animal models of atherosclerosis to evaluate the applicability of these findings in a clinical setting.
The groundbreaking research - conducted by NOVA Medical School, led by Otília Vieira but with collaboration with other NMS groups and also University of Coimbra, Coimbra, UCL Institute of Ophthalmology, University of Sheffield and National Heart, Lung, and Blood Institute (NHLBI) - not only advances our understanding of the molecular mechanisms underlying atherosclerosis but also holds promise for the development of targeted interventions to combat this prevalent and devastating disease. With cardiovascular diseases remaining the leading cause of death globally, this study represents a significant step forward in the ongoing battle against atherosclerosis.

André Marques, Neuza Domingues, Otília Vieira, Inês Ferreira & José Ramalho
The study, titled "Oxidized cholesteryl ester induces exocytosis of dysfunctional lysosomes in lipidotic macrophages," was recently published in the scientific journal Traffic and can be read here:
Domingues N, Marques ARA, Calado RDA, Ferreira IS, Ramos C, Ramalho J, Soares MIL, Pereira T, Oliveira L, Vicente JR, Wong LH, Simões ICM, Pinho E Melo TMVD, Peden A, Almeida CG, Futter CE, Puertollano R, Vaz WLC, Vieira OV. Oxidized cholesteryl ester induces exocytosis of dysfunctional lysosomes in lipidotic macrophages. Traffic. 2023 May 2. doi: 10.1111/tra.12888. Epub ahead of print. PMID: 37129279.
Otília Vieira
Principal Investigator
André Marques
Assistant Researcher
Inês Ferreira
PhD student
Rita Calado
Post-Doctoral Researcher
Winchil Vaz
Senior Scientist
Telmo Pereira
Microscopy Facility Manager
Cláudia Guimas Almeida
Principal Investigator