- halt-RONIN (halt-chROnic INflammation)
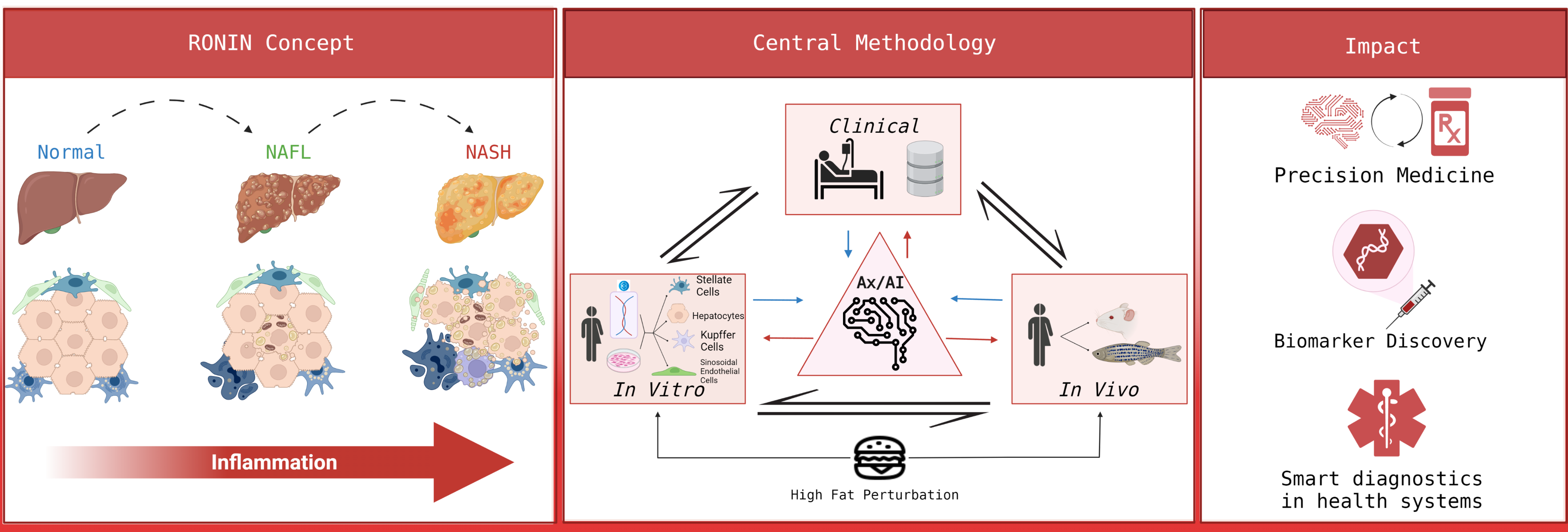
Funding by Horizon Europe (HORIZON-HLTH-2022-STAYHLTH-02/101095679)
Collaboration with Prof. Javier Cubero (Complutense University of Madrid - coordinator)
Know more here.
- Prospective European Drug-Induced Liver Injury Network

The project regards an EU funded Cost Action which addresses a serious health problem in Europe namely drug induced liver injury or DILI. The objectives of the PRO-EURO-DILI-NET Cost Action are to create a unique, co-operative, interdisciplinary European-based DILI network of stakeholders to co-ordinate efforts in DILI, to facilitate bi-directional exchange of discovered knowledge and generated hypotheses among different disciplines, and to promote clinically impactful knowledge discovery and its translation into clinical practice.
This Action will:
-
- Harmonize efforts for in-depth DILI phenotyping and bio-sample repository and coordinate pre-funded database/repository studies to aggregate a large number of DILI cases in a standardized manner (WG1).
- Establish a strategy for development, validation and performance of DILI novel biomarkers and explore multifactorial DILI risk modifiers in clinical data sets using novel approaches for future precision medicine (WG2).
- Facilitate clinically impactful knowledge discovery by introducing biological variations and the complexity (i.e., multi-cellular/multiorgan systems) into toxicological experiments to assess hepatotoxicity to guide future drug safety testing (WG3).
- Define criteria and establish endpoints to measure efficacy on novel interventions in DILI (WG4).
- Draft policy recommendations about near-patient testing tools.
The network will promote and coordinate a highly translational and innovative research program in Europe and beyond with the ultimate goal to pre-empt and prevent DILI, develop innovative therapeutic approaches that could improve clinical outcomes and enhance public awareness, while developing a forum for knowledge exchange and training of young European researches.
Michel Kranendonk, the head of the Xenobiotic Metabolism lab, is member of the Management Committee of this Cost Action as well as member of the Workgroup 3.
Website: https://proeurodilinet.eu/
Open-access publication:
ADVANCED PRECLINICAL MODELS FOR EVALUATION OF DRUG INDUCED LIVER INJURY - CONSENSUS STATEMENT BY THE EUROPEAN DRUG-INDUCED LIVER INJURY NETWORK [PRO-EURO-DILI-NET] Checa et al. (2021) J Hepatol https://doi.org/10.1016/j.jhep.2021.06.021
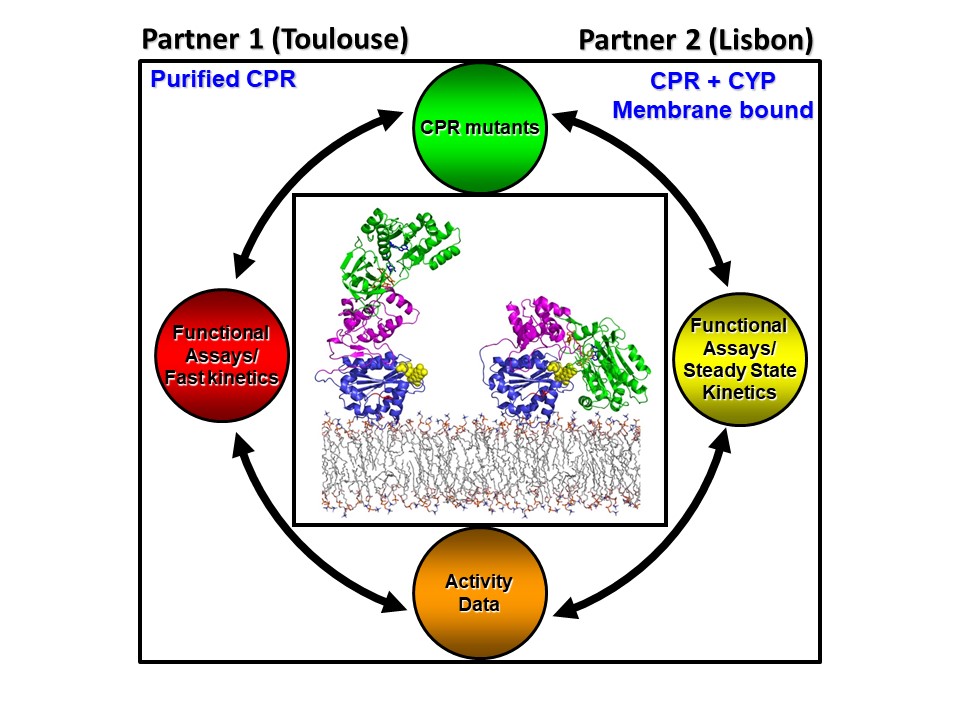
- Domain Dynamics and Control of Electron flux (DODYCOEL)
NADPH cytochrome P450 oxidoreductase (CPR) is a diflavin protein which is the obligatory electron donor of CYP and non-CYP enzymes, which are involved in many physiological important processes. CPR, like all diflavin electron transfer (ET) enzymes, is a multidomain protein which undergoes large conformational changes in its ET function. CPR exists in a conformational equilibrium, between a closed (electron acceptor (NADPH) conformation and an open electron donation (interaction with redox partner) conformation. At the start of the project there were indications that both intrinsic (CPR determinants) and extrinsic (membrane environment and redox partners) control CPR’s open-closed dynamics, however several of these critical parameters were not analyzed.
The project had as goal to address these issues by:
-
- Characterizing the different conformational states of CPR populated during its interaction with various natural electron acceptors (CYP)
- Understanding and identifying how CYP interaction controls and modulates the different steps of the CPR enzymatic cycle, ultimately defining a perfect coupling scheme between the two enzymes;
- Describing the CPR molecular determinants that change the structural organization and conformational equilibrium to formalize how critical structural elements affect CPR function.
The collaborative project between Lisbon and Toulouse (INSA) addressed these issues using both soluble purified (Partner 1, Toulouse) and membrane bound CPR forms (Partner 2 Lisbon).
Several critical residues in a specific segment of the CPR protein, the hinge region could be identified in the open-closed dynamics of CPR, in which ionic strength seems to play a role. This extensive protein dynamics is responsible for the formation of an ensemble of open CPR conformers, by which CPR can interact with so many structurally different redox partners. Moreover, it was demonstrated that each redox partner (i.e. CYPs) interact with CPR via the use of specific biding-motifs of the interaction surface, the FMN domain, in an isoform specific manner. Additionally, we found that the substrate bound by CYP influences this interaction.
Team Members:
Bernardo Brito Palma, Bruno Costa Gomes, Francisco Esteves, Diana Campelo
Esteves F, et al. Interaction Modes of Microsomal Cytochrome P450s with Its Reductase and the Role of Substrate Binding. Int. J. Mol. Sci. 2020 Sep 11;21(18):6669. doi: 10.3390/ijms21186669
Esteves F, etal. The Role of the FMN-Domain of Human Cytochrome P450 Oxidoreductase in Its Promiscuous Interactions with Structurally Diverse Redox Partners. Frontiers Pharmacol. 2020 Mar 18;11:299. doi: 10.3389/fphar.2020.00299
Campelo D, et al. Probing the Role of the Hinge Segment of Cytochrome P450 Oxidoreductase in the Interaction with Cytochrome P450. Int. J. Mol. Sci. 2018 Dec 6;19(12):3914. doi: 10.3390/ijms19123914
Quast RB. et al., Accurate Determination of Human CPR Conformational Equilibrium by smFRET Using Dual Orthogonal Noncanonical Amino Acid Labeling. Chembiochem. 2019 Mar 1;20(5):659-666. doi: 10.1002/cbic.201800607
Esteves F, et al. Human cytochrome P450 expression in bacteria: Whole-cell high-throughput activity assay for CYP1A2, 2A6 and 3A4. Biochem Pharmacol. 2018 Dec;158:134-140. doi: 10.1016/j.bcp.2018.10.006
Campelo D, et al. The Hinge Segment of Human NADPH-Cytochrome P450 Reductase in Conformational Switching: The Critical Role of Ionic Strength. Frontiers Pharmacol. 2017 Oct 30;8:755. doi: 10.3389/fphar.2017.00755
Esteves F, et al. The Central Role of Cytochrome P450 in Xenobiotic Metabolism—A Brief Review on a Fascinating Enzyme Family. J.Xenobiot. 2021, 11, 94–114. https://doi.org/10.3390/jox11030007
