Cristina Escrevente and Liliana Bento-Lopes are first authors of a work performed in the Membrane Traffic in Disease Lab and led by Duarte Barral

From left to right: Liliana Bento-Lopes, Cristina Escrevente, Duarte Barral
This research, within the scope of the Horizon 2020 Twinning LYSOCIL project, was published on the Journal of Cell Science in Open Access under the title “Rab11 is required for lysosome exocytosis through the interaction with Rab3a, Sec15 and GRAB”. Read it here.
What discoveries led you to the research described in your publication?
Lysosomes have long been regarded as the compartment used by cells to dispose of their waste. However, we now know that these intracellular compartments with digestive properties work like a recycling station. Lysosomes can also fuse with the membrane that limits the cell (i.e. plasma membrane), secreting their contents to the extracellular space, in a process called lysosome exocytosis. This process is crucial for several cellular functions, including plasma membrane repair, which is required, for example, by muscle cells to recover after physical exercise.
Lysosome exocytosis is known to occur in almost all cell types, but the molecular machinery involved in this process is poorly understood. In a previous study, done in collaboration with the group of Dr. Otília Vieira, also from CEDOC, we have discovered that a protein named Rab3a is required for lysosome exocytosis and plasma membrane repair (Encarnação et al., J. Cell Biol., 2016). However, other proteins from the same family were also found to be important for lysosome exocytosis and, in this study, we turned our attention to one called Rab11.
What were you trying to understand and what is the main discovery of this work?
We want to understand how lysosome exocytosis is regulated. Lysosomes contain digestive enzymes and therefore, their fusion with the plasma membrane must be tightly regulated since in this process, the enzymes are released from the cell.
In this study, we discovered new regulators of lysosome exocytosis, namely Rab11 and other molecules that interact with it, establishing a cascade that links Rab11 to Rab3a, a molecule previously described by us to regulate this process.
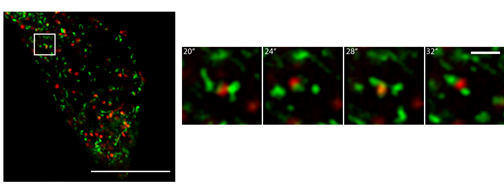
Live imaging of human cell lines showing colocalization of Rab11 - fused with green fluorescent protein (GFP) - and the lysosome – in red fluorescent staining. Image copyright belongs to Journal of Cell Science
Why is this important?
By describing the role of Rab11 and associated proteins in lysosomal exocytosis, we are a step closer to more comprehensively understand how cells regulate such an important process required for their maintenance and survival.
Lysosome exocytosis is particularly important for plasma membrane repair in tissues susceptible to mechanical or ischemic stress, such as skeletal and cardiac muscle. The fusion of lysosomes with the plasma membrane also leads to the release of digestive enzymes and this process can be subverted by cancer cells to invade adjacent tissues and form metastases.
Shedding light on the molecular machinery that regulates lysosome exocytosis can help identify new therapeutic targets for several diseases such as muscular dystrophy and myopathies, as well as cancer.
Can you use an analogy to help us understand your work?
If we cut our skin, we bleed. But the bleeding is rapidly stanched through blood clotting mediated by platelets. Lysosomes behave more or less like this. They can sense a leakage in the limiting membrane of the cells and quickly move to the place where the membrane is damaged, to stop the leakage. Therefore, it is very important to understand how this process is regulated, not only to modulate it in the case of membrane damage, but also to understand how cancer cells subvert this mechanism to their advantage and how lysosome exocytosis can be involved in musculoskeletal disorders.
What questions remain to be asked?
This work provides evidence that Rab11 is a key regulator of lysosome exocytosis, together with other binding partners. But can we use this knowledge to modulate lysosome exocytosis to control cancer cell invasion and tumor progression? And can we use this knowledge to improve plasma membrane repair in the case of musculoskeletal diseases? We still have much more to understand about the many roles of lysosomes and how important they are in cell physiology and several pathologies.
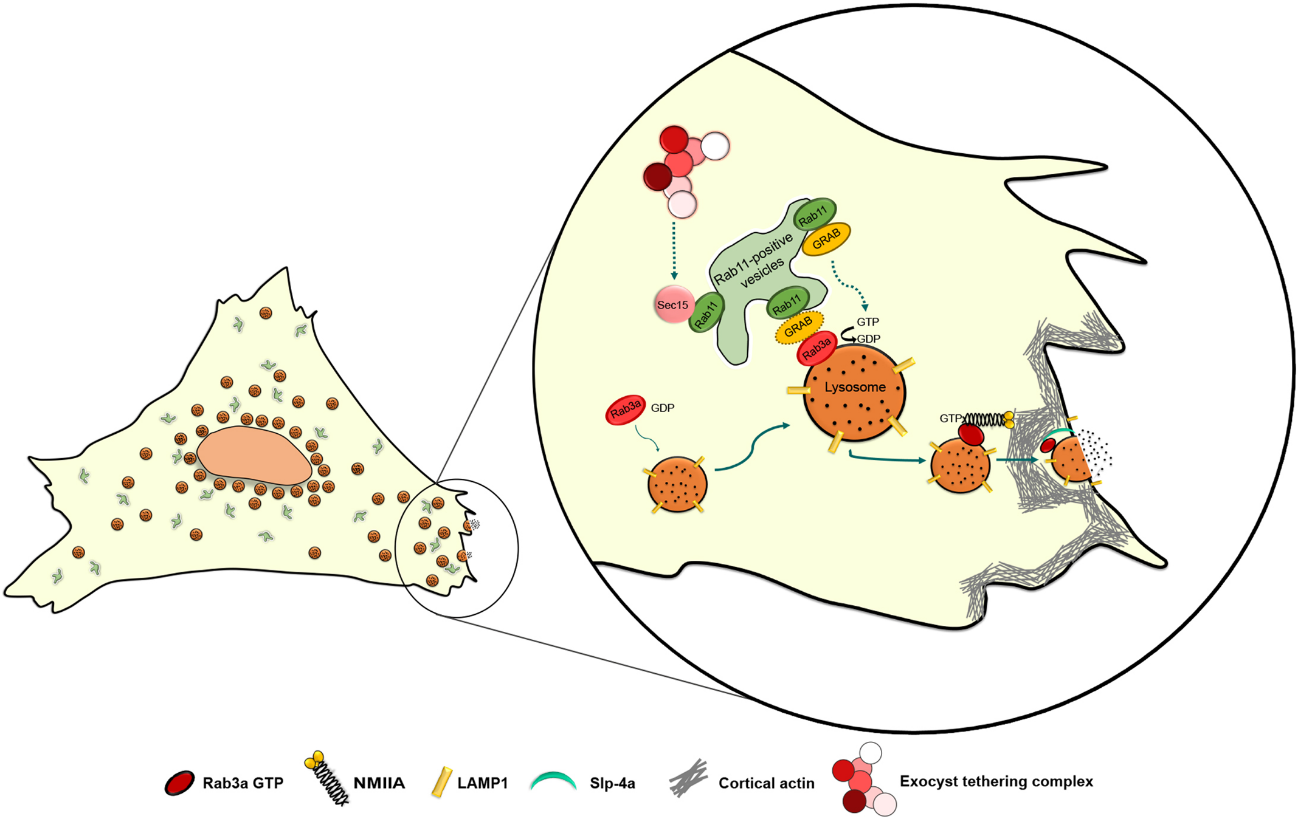
Proposed mechanism for the role of Rab11 in Ca2+-triggered lysosome exocytosis. Rab11 binds directly to Sec15, a subunit of the exocyst complex. Sec15 might function independently of the exocyst complex, facilitating the transport of Rab11-positive vesicles along the actin cytoskeleton via the interaction with myosin V (not shown). Rab11-positive vesicles are derived from the endocytic recycling compartment (ERC) or the Golgi (post-Golgi vesicles). Rab11-positive vesicles interact with Rab3a-positive lysosomes, possibly to deliver cargo proteins required for lysosome exocytosis, e.g. GRAB. We hypothesize that GRAB mediates the interaction between Rab11 and Rab3a. GRAB could then catalyze the conversion of Rab3a from a GDP-bound to GTP-bound state, thereby activating it, which recruits NMIIA and Slp-4a, allowing the correct positioning of lysosomes before lysosome exocytosis. Image copyright belongs to Journal of Cell Science